
Two agencies oversee food inspection in the US, but who regulates what? It's complicated.
This story originally appeared on FoodReady and was produced and distributed in partnership with Stacker Studio.
Two agencies oversee food inspection in the US, but who regulates what? It's complicated.
On your most recent trip to the grocery store, you may have checked your packaged salad for withering leaves or your apples for bruises.
Yet when it comes to more serious food safety concerns, like contracting salmonella from your favorite frozen chicken pot pie or contracting norovirus from contaminated fruit, Americans largely depend on two regulatory bodies: the Food and Drug Administration and the United States Department of Agriculture. These organizations perform inspections during the food production process to keep consumers safe.
The organizations operate with different policies, procedures, and inspection schedules, and the reality on the ground can be confusing. FoodReady compared the USDA and FDA's food safety responsibilities and inspection budgets, and explored why two distinct agencies oversee the nation's food supply chain.
Broadly speaking, the USDA is responsible for ensuring the safety and health of American agricultural products. The FDA, meanwhile, regulates labeling on animal feed, drugs, dietary supplements, food, and several other meat- and poultry-related products.
But the lines between who is monitoring what—and why—are often murky.
Take frozen pizza, for example. If you pick up a pepperoni pizza from the frozen foods section of your supermarket, the USDA has inspected it at three different points: the slaughterhouse, the pepperoni factory, and the pizza factory, according to a report by Eater. But if you choose a plain cheese pizza, you're buying a product that was likely only inspected once by the FDA when the pizza company added the nutrition label.
The USDA inspects sausages, but the FDA inspects the casings in which the sausages are wrapped. Freshly picked raw fruits and vegetables fall under the USDA's purview, but once they are processed—mashed into apple sauce, squeezed into juice, or mixed with other ingredients—the FDA takes over.
These splits don't necessarily reflect the level of risk inherent to each type of food, either. For example, fish largely fall under the FDA's jurisdiction, but due to the 2008 Farm Bill, the USDA now inspects catfish.
"Catfish are no less safe than salmon or shrimp," Dr. Richard Raymond, former Under Secretary for Food Safety for the Food Safety and Inspection Service, the USDA's enforcement arm, told Food Safety News. But catfish are now the only seafood to undergo daily inspections.
How can incredibly similar products with the same risks undergo different inspection processes? It's complicated—but the short answer is American bureaucracy.
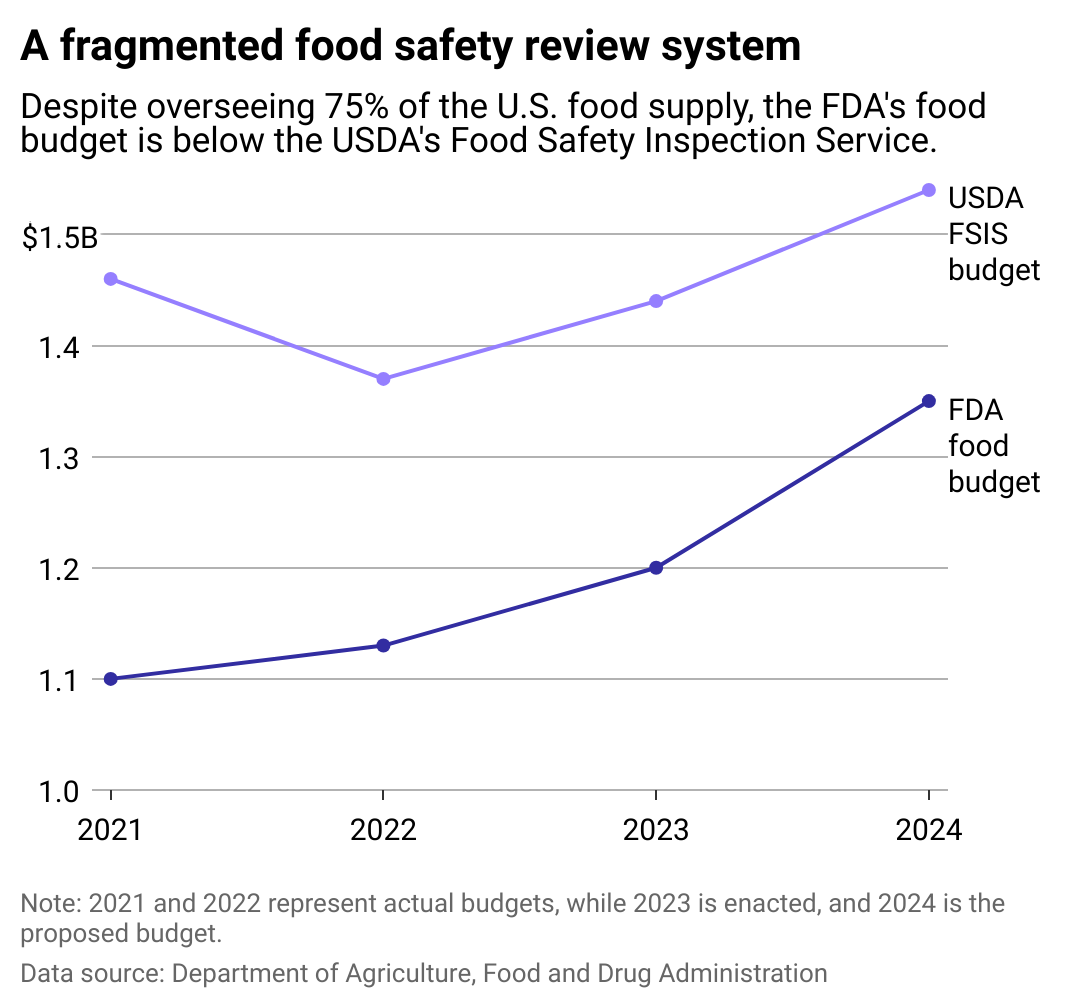
A tale of two departments
The U.S. government has been using chemical analysis to track the safety of agricultural products since 1848. The USDA took over this responsibility in 1862 upon its founding, establishing a centralized organization for monitoring the American food supply and various other duties.
The FDA—called the Bureau of Chemistry until 1930—gained the power to enforce regulations in 1906 with the passage of the Pure Food and Drugs Act, which made selling degraded or misbranded food and drugs illegal. Finally, the USDA formed a division called the Food Safety and Quality Service in 1977; it was renamed the Food Safety and Inspection Service in 1981.
Today, the FDA oversees 78% of the U.S. food supply. Yet its food budget is lower than the USDA's FSIS, which supervises a much smaller percentage of the food Americans consume. While the USDA proactively inspects farms and facilities (in some cases daily), the FDA only inspects food in its jurisdiction every three to five years.
That means the FDA primarily relies on customers tipping them off about problems or on manufacturers and retailers to recall potentially unsafe foods voluntarily—opening the door to serious issues.
People in 10 states in November 2021 got sick after eating spinach; three people experienced kidney failure. By the time the FDA traced it back to the spinach, Politico reported, the season was over, and the production facilities were empty, rendering it impossible to trace the exact source of contamination. Was it the soil? The water? Something else? No recall was initiated.
The FDA faces more than a funding issue. Industry insiders say the organization is known to be sluggish and incapable of responding promptly, citing an unclear organizational hierarchy and risk-averse departmental culture unwilling to advance policy.
The administrations of Barack Obama and Donald Trump attempted to pass legislation to restructure them while former leaders of both departments have jointly called for them to be consolidated and streamlined.
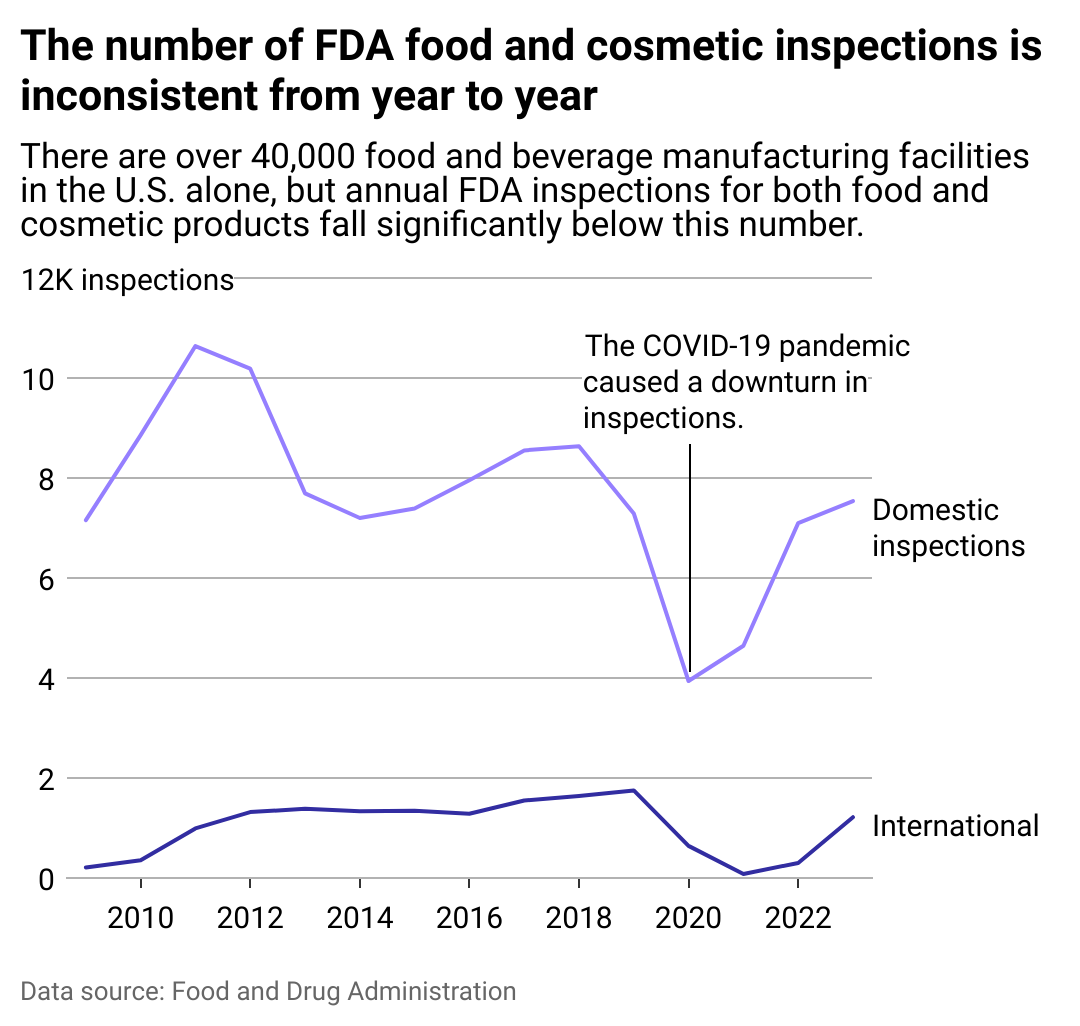
FDA inspections fall short
The Food Safety Modernization Act, signed into law in 2011, requires the FDA to inspect facilities once every five years—or once every three years for high-risk facilities. But even with those longer timelines, the FDA struggles to keep up with its work.
Janet Woodcock, the FDA's principal deputy commissioner, told Politico in a 2022 interview that while the food division was a priority, it was chronically underfunded. This could be due in part to the fact that life-saving medications tend to get more attention and resources than slower (but also life-saving) efforts to, for example, reduce the sodium levels in processed food or ensure vegetables aren't grown using contaminated water.
While the USDA Food Safety Inspection Service receives more funding, it has its problems, too. Food safety microbiologist Phyllis Entis wrote in an op-ed for Food Safety News that the USDA has two goals that can cause a conflict: The organization is supposed to ensure food safety while promoting U.S. agricultural products worldwide.
"This is akin to having the quality assurance department of a food company report to the head of the marketing department," Entis wrote. "We have seen the consequences of this conflict most recently in the FSIS draft proposal to allow as much as one Salmonella per gram of chicken in raw, breaded stuffed chicken products."
Entis supported a proposal by advocate Bill Marler to "Get the F out of FDA" and separate entities. One would be responsible for cosmetics and medical devices, and the other for food and human nutrition.
Entis takes Marler's suggestion a step further, however, and preaches that the U.S. should follow the lead of Canada, Australia, and New Zealand and host all food safety issues under one agency.
"But it's more than just about who inspects what foods," Frank Yiannas and Mindy Brashears, former senior food leaders at the FDA and USDA, respectively, wrote in their joint op-ed for Food Safety News.
"More importantly, it's about how do we take a more modern, risk-based, and data-driven approach to how we regulate the safety of our food supply. Why is a pepperoni pizza subjected to continuous inspection, yet something as critical as powdered infant formula, at best, inspected only once per year."
Story editing by Nicole Caldwell. Copy editing by Kristen Wegrzyn.



