15 ways doctors are now treating COVID-19
Doctors have far more options to try to treat COVID-19 patients now than they did in late winter and early spring of 2020. That often means buying enough time for patients' immune systems to fight off the virus on their own. It also means reducing the number of patients who advance to the point that they require mechanical ventilation, and above all, reducing the number of deaths from the virus.
Stacker used resources from the Food & Drug Administration (FDA), the Harvard Medical School, and other scientific sites to compile a list of major treatments and strategies that doctors across the country are using to mitigate the effects of COVID-19. These treatments cover a huge range, from the "common sense" remedies to ease mild cases at home to antibodies, steroids, antivirals, and physical interventions like ventilators. Many have been approved on an emergency basis by the FDA. Some, like promising corticosteroid dexamethasone, were already approved for other instances of respiratory disease.
Some of the major ways to "treat" COVID-19 come in the form of prevention—as the saying goes, an ounce of prevention is worth a pound of cure. For many of us, that ounce of prevention is as simple as frequently washing our hands, wearing masks whenever necessary, and social distancing. No matter how good the treatment for COVID-19 gets, nothing beats not getting it in the first place.
Keep reading to learn about 15 ways doctors are now treating COVID-19.

Increased testing
Testing people frequently, including when they have symptoms and when they have a credible fear of exposure, can help medical officials trace how the virus is moving through a geographic area. Frequent testing also helps ensure that numbers continue to fall.

Rapid review of new scientific research
A typical, peer-reviewed journal takes at least a few months but often closer to a year to cycle submitted papers all the way to publication—and that’s fine for most conditions. In 2020, the world saw scholarly research get a green light and a steady push from reviewers who are almost waiting by the phone. That means faster validation of vaccine experiments, for example, or studies of the efficacy of virus-killing UVC light.

Doctor support networks
Doctors keep up with the literature within their specialties, both for ethical reasons and to fulfill continuing education requirements. But COVID-19 treatment is happening in real-time around the world, and doctors are meeting in virtual groups to compare notes on what’s working and what’s not.
Remdesivir (antiviral drug)
Remdesivir is an IV antiviral drug that has slowed the replication of the COVID-19 virus in clinical trials. That means patients have a lower viral load for a longer time, which scientists say could slow any worsening of the virus. Patients in the meantime have more time to be less sick, increasing their odds of recovery.
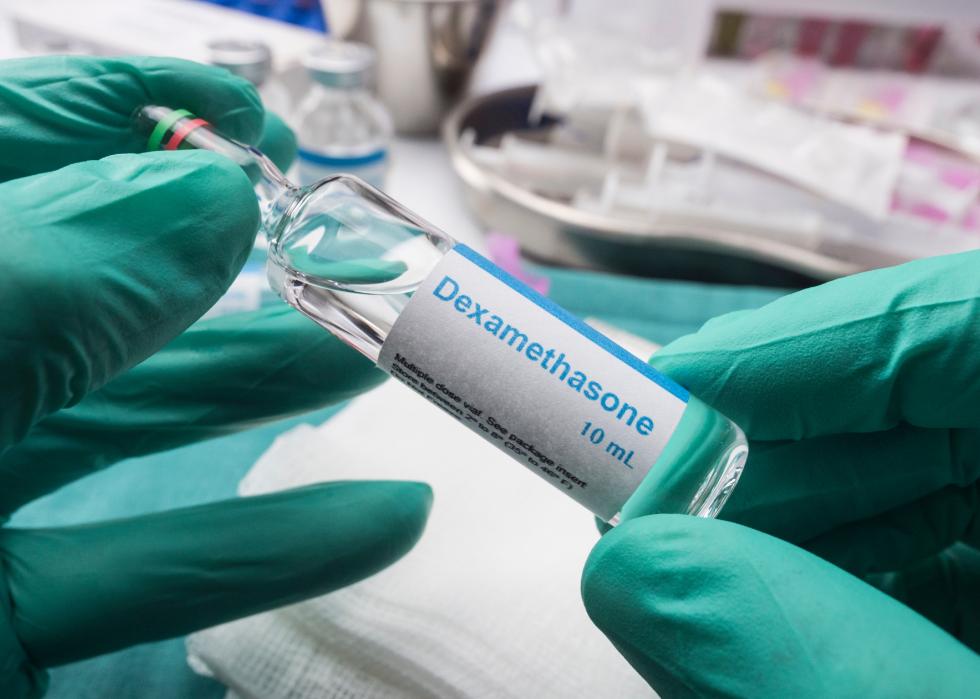
Dexamethasone (anti-inflammatory drug)
Dexamethasone is a corticosteroid, meaning it's a hormone push that triggers anti-inflammatory changes in your body. It's already widely used around the world for treating respiratory distress in premature babies, for example. And for patients with severe COVID-19 who are on ventilators, dexamethasone can reduce mortality by as much as one-third.
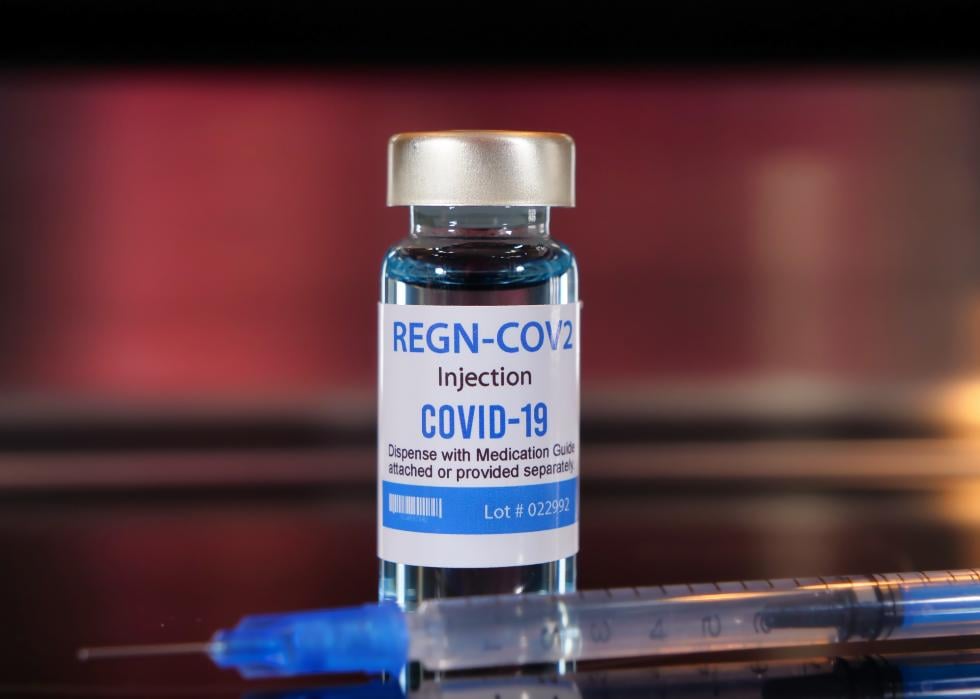
Regeneron’s monoclonal antibody treatment
Pharmaceutical company Regeneron made news in September 2020 with promising research about its “COVID-19 cocktail” antibody treatment. This is reportedly what former President Donald Trump received during his stay at Walter Reed National Military Medical Center. The U.S. government agreed to purchase up to 1.25 million doses of Regeneron in January 2021.

Bamlanivimab (monoclonal antibody treatment)
The FDA has authorized the emergency use of Eli Lilly’s antibody treatment bamlanivimab, which, they say, works best on patients who are still in the early stages of COVID-19 and not hospitalized or receiving ventilation support. In a study, just 3% of patients given bamlanivimab after contracting the virus ended up in the hospital—compared with 10% of patients given a placebo.
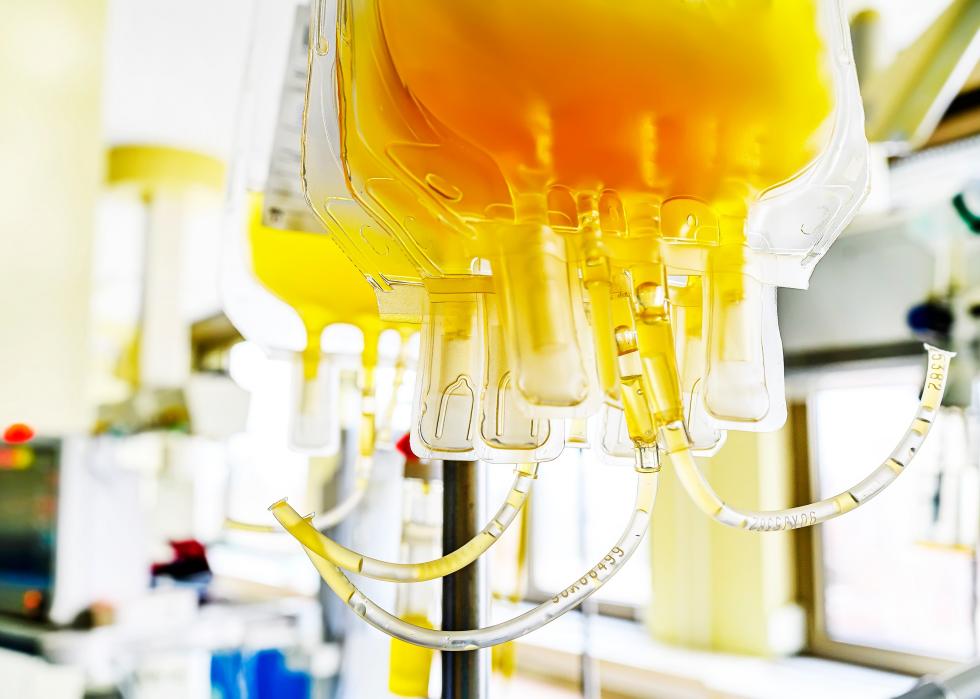
Convalescent plasma
The FDA issued an emergency use authorization of a potential treatment in which hospitalized COVID-19 patients receive plasma donated by people who have recovered from the virus. Replacing plasma is already a safe, low-risk procedure, and scientists believe the plasma of recovered patients could boost recovery rates for hospitalized patients. At the very least, they say, it’s worth trying.

Ventilators
Ventilators are mechanical breathers with a twofold effect. First, they keep patient airways open and able to constantly take in and expel air. Second, and just as important for people with COVID-19 lung impairment, ventilators physically push the air into the lungs to make sure they’re fully inflating with fresh, oxygenated air.
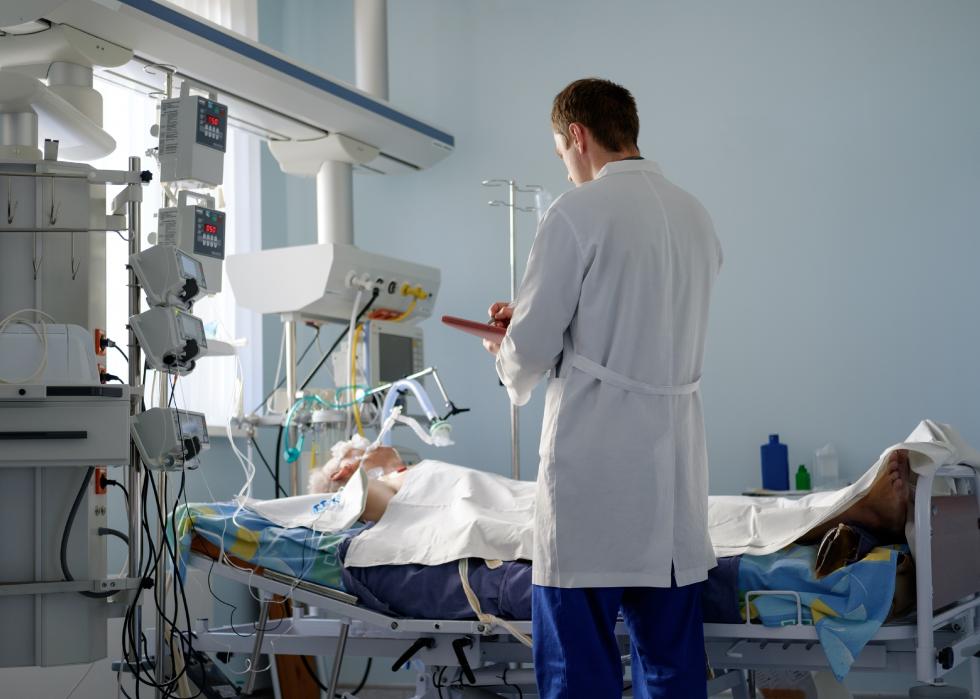
Prone positioning
Prone positioning is the industry term for placing patients facedown on their stomachs. Doctors say this helps reposition the lungs, opening up new lung terrain that isn't accessible when patients lie on their back. This can rapidly increase the key statistic of oxygenation of the blood.

Respiratory assistance
Ventilators aren’t the only kind of respiratory aid that can help patients who are hospitalized with COVID-19. Other FDA-approved equipment ranges from muscle stimulators to nerve stimulators designed to work in conjunction with mechanical ventilation. That can mean better results for patients who have access to multiple modes of treatment.
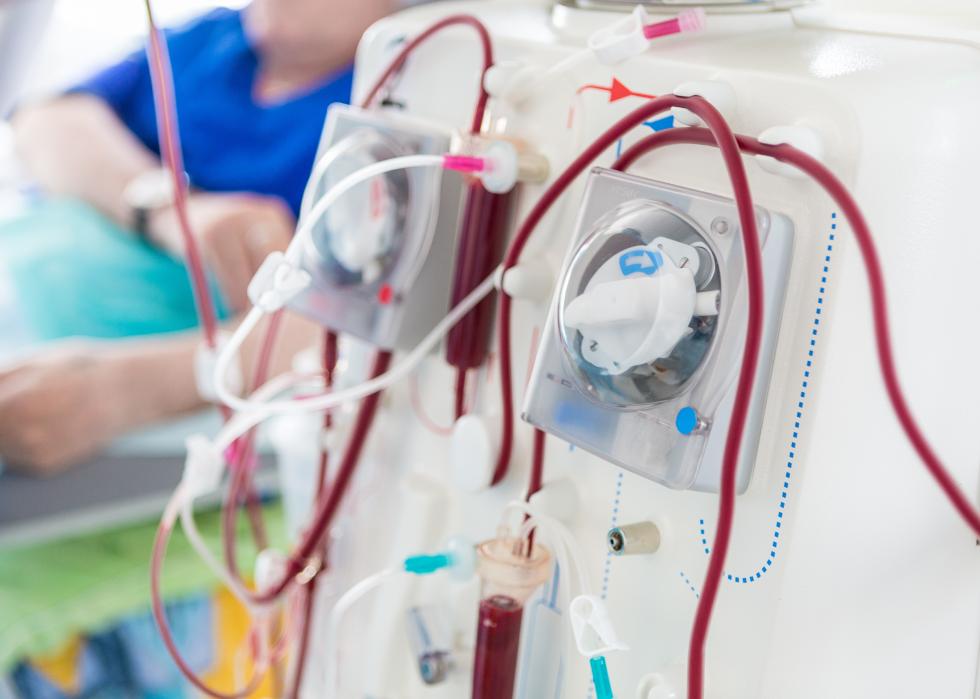
Blood purification
The FDA has authorized emergency usage of a procedure called blood purification, which is just what it sounds like: blood is circulated through an external filtering device that reduces disease agents as well as runaway immune response chemicals that can harm patients.
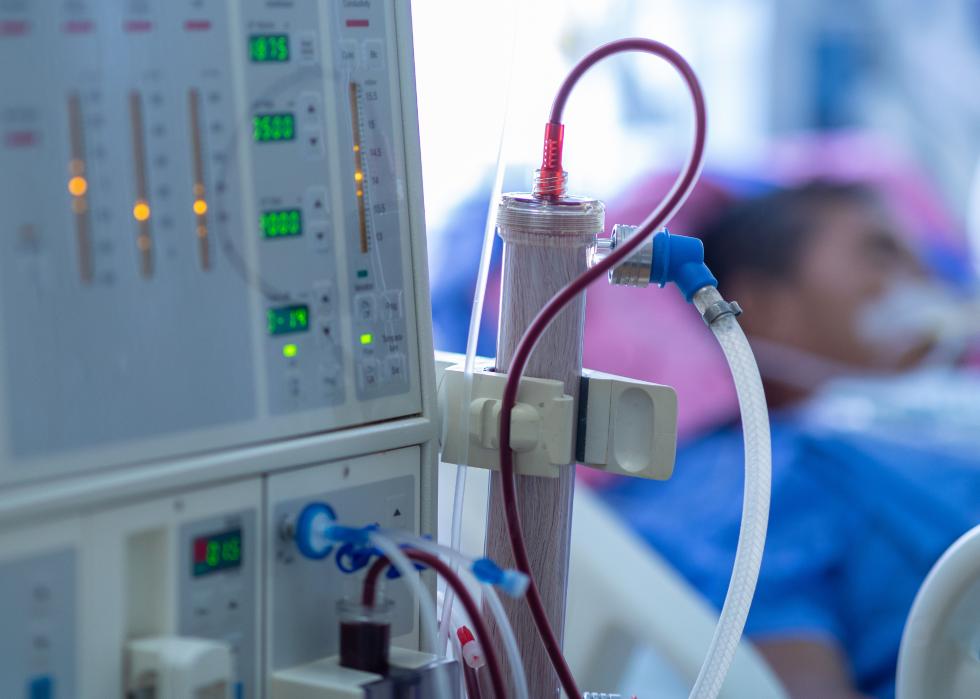
Continuous renal replacement therapy
In severe cases, COVID-19 can cause organ failure, including of the kidneys. Because of that, the FDA has approved the use of dialysis technology to help support patients with diminished kidney function.

At-home treatment
For people with mild symptoms of COVID-19, health care professionals advise isolating at home to recover—the same as you would for a confirmed case of flu, but with even more social precautions. That means staying hydrated, taking over-the-counter pain relievers or fever reducers like Tylenol, and a continuous supply of surface-disinfecting cleaning products.
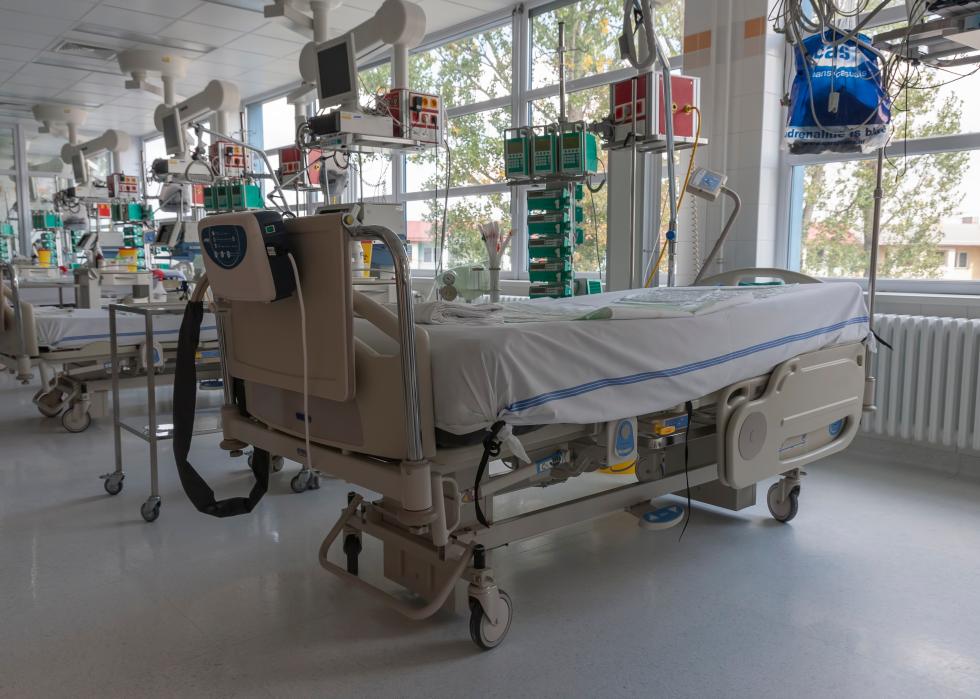
Successful treatment requires hospital capacity
When hospitals are at capacity, the risk of death for COVID-19 patients can be close to double that faced when ICUs are less busy. The best way to keep more patients out of hospitals is to use every precaution available, including masks, social distancing, and frequent hand-washing.



