Naloxone is now approved for over-the-counter use. What are the hurdles to accessing it?
Naloxone is now approved for over-the-counter use. What are the hurdles to accessing it?
On March 29, 2023, the FDA approved Narcan, a brand name of naloxone nasal spray used to immediately treat those experiencing an opioid overdose, for over-the-counter sale. The decision was made in an effort to make naloxone treatments more accessible to the public as drug-involved overdose deaths have been rising steadily since 2018.
Despite over-the-counter approval for Narcan, there are still many obstacles that those seeking to have naloxone on hand will have to overcome. Stacker investigated the hurdles to accessing naloxone over the counter using a variety of news and medical sources.
For now, the approval only impacts the dispensing of Narcan specifically, but it is likely that other naloxone nasal spray treatments will be approved for over-the-counter use in the wake of this decision. The complete switch from Narcan being a behind-the-counter drug to an over-the-counter offering across the country will likely take months as all Narcan packaging, advertisements, and store placements must be changed.
Prices may be prohibitively high.
For generic naloxone, a dose is often $20 to $40, and name-brand Narcan can run anywhere from $130 to $140 for a two-dose kit. Prior to the over-the-counter approval of naloxone, most insurance plans would cover the cost of the treatment. However, some fear that the price of the drug will rise now that it can be obtained without a prescription or approval by a pharmacist, which, if such fear is realized, would mean people who are in most need of naloxone will be unwilling or unable to pay for it.
Though the naloxone nasal spray is the only form of the drug that has been approved for over-the-counter use, there are two other methods for administering naloxone. The first is liquid naloxone administered by injection with a syringe. The drug comes in a glass vial and can be given in larger doses than the nasal spray. This requires some medical training to administer and is given by prescription only. The second is the naloxone auto-injector, the original method developed for administering naloxone outside of a medical setting. The drug is a preset dose and operates similarly to an EpiPen, as it is used to inject naloxone into the muscle or under the skin. It is likely that these treatments will continue to be covered by insurance.
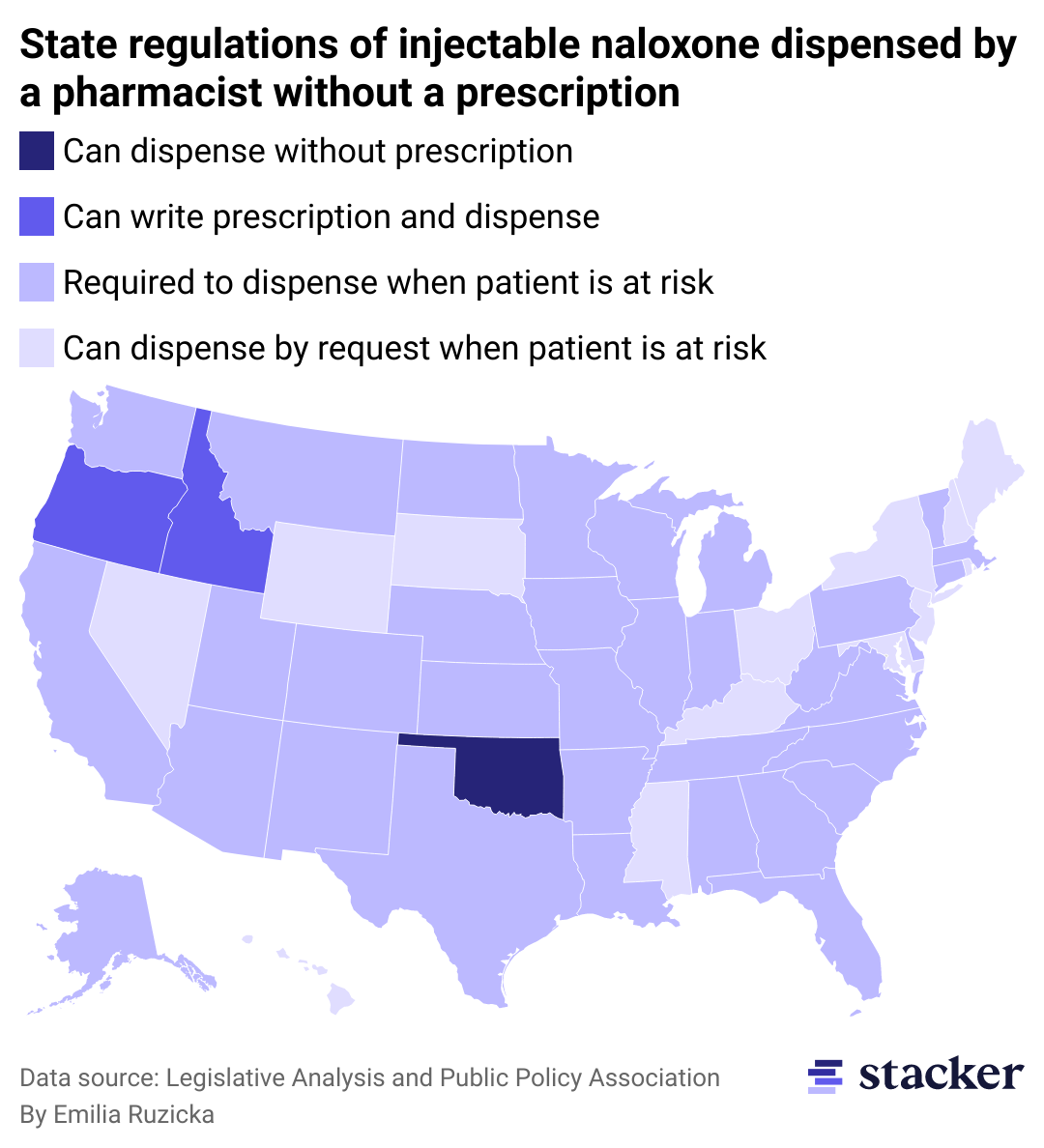
Not all pharmacies will carry naloxone.
In 2021, there was a widespread naloxone shortage in the U.S. due to one of the primary manufacturers of naloxone, Pfizer, experiencing supply chain issues in its manufacturing process for the injectable version of the drug. Although this acute shortage has since been alleviated, the number of people who would benefit from having naloxone on hand has always outweighed the supply. As a result, it can be difficult for pharmacies to keep naloxone in stock, no matter what the demand is in their community.
In addition, the stigma around opioid use and the risk of overdosing means that some people don't receive doses of naloxone to have at home, even if it is prescribed or readily available to them. This may be because they feel embarrassed to fill the prescription or fear that there will be negative consequences if they are found in possession of naloxone due to its association with drug use.
There is also evidence to suggest that some people who use drugs don't keep doses of naloxone around during periods of sobriety because they believe they are no longer at risk. Some pharmacists have expressed fears of dispensing naloxone because of stigmas against the "clientele who would frequent the store" and the belief that naloxone gives people who overdose a "free pass," despite proof that naloxone use reduces overall harm.
Though cheaper injections have been available behind the counter, they can be difficult or scary to administer
Even before the naloxone nasal spray was approved for over-the-counter use, all 50 states had some provision in place to allow for naloxone access without a prescription from a doctor. These regulations no longer apply to naloxone nasal spray with the FDA's over-the-counter approval, but injectable forms of naloxone are still required to be dispensed by a pharmacist in some way. These injectable doses are cheaper than the nasal spray—often by half or more—and are the most common form given out by nonprofit and drug addiction aid groups due to their relative affordability.
The experience of administering naloxone nasal spray is relatively quick and painless compared to injectable versions. With liquid naloxone from a vial, the person administering the medication must pull the proper dose from the glass packaging using a syringe before injecting the recipient. The process puts the user at risk of providing the wrong dose, breaking glass, or snapping the needle of the syringe and takes precious seconds during which the person who overdosed may experience complications.
The naloxone auto-injector is easier to use because it is pre-loaded with the dosing but still requires slightly more preparation than the nasal spray prior to administering the medication. Though—outside of an overdose situation—these steps to administer injectable naloxone may appear negligible, they can often be obstacles that make or break whether a bystander is able to provide help to someone suffering from an overdose.



