Zepbound vs. Wegovy: Comparing weight loss drugs
Zepbound vs. Wegovy: Comparing weight loss drugs
Zepbound (tirzepatide) and Wegovy (semaglutide) are FDA-approved injectable medications revolutionizing weight management. Both belong to the incretin mimetic drug class but differ in their mechanisms: Zepbound activates both GLP-1 and GIP receptors, while Wegovy targets only GLP-1. These medications help regulate appetite, slow gastric emptying, and promote satiety, making them powerful tools for chronic weight management in qualified patients.
In this comparison between Wegovy versus Zepbound, SaveHealth, a prescription discount/savings card website, explores the differences between GLP-1 agonists.
Mechanism of action: Zepbound versus Wegovy comparison
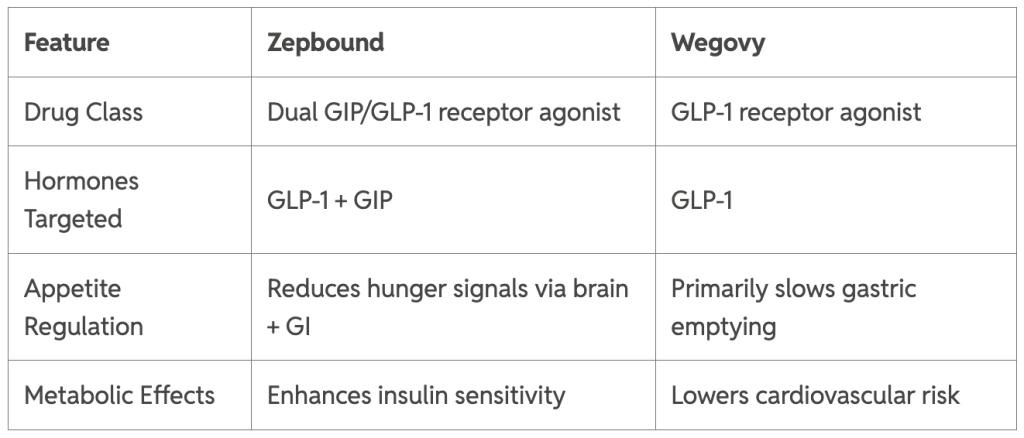
Zepbound's dual-action approach may lead to more significant weight loss by simultaneously targeting two hunger-regulating pathways.
Efficacy and weight loss outcomes
Clinical trials demonstrate distinct results:
- Zepbound: Participants lost 20.2% of body weight on average (50.3 lbs) over 72 weeks in SURMOUNT-5 trials.
- Wegovy: Average weight loss of 13.7% (33.1 lbs) in similar studies.
Key secondary outcomes:
- 64.6% of Zepbound users achieved ≥15% weight loss vs. 40.1% with Wegovy.
- Zepbound reduced waist circumference by 7.2 inches vs. Wegovy's 5.1 inches.
New England Journal of Medicine-published SURMOUNT-5 trial confirmed Zepbound's superior weight reduction in a head-to-head comparison.
FDA-approved indications
Zepbound
- Adults with BMI ≥30 (obesity).
- Adults with BMI ≥27 plus at least one weight-related comorbidity (symptomatic heart failure with preserved ejection fraction, metabolic dysfunction-associated steatohepatitis).
- Moderate-to-severe obstructive sleep apnea management with obesity.
Wegovy
- Adults and adolescents (12-plus) with obesity (BMI ≥30).
- Overweight adults (BMI ≥27) plus at least one weight-related comorbidity (symptomatic heart failure with preserved ejection fraction, noncirrhotic metabolic dysfunction associated with moderate to advanced fibrosis).
- Cardiovascular risk reduction in adults with heart disease.
Wegovy holds broader pediatric approval, while Zepbound offers OSA-specific indications.
Dosage and administration
Zepbound Titration
- Start: 2.5 mg weekly for four weeks.
- Increase by 2.5 mg monthly.
- Maintenance: 5 mg, 10 mg, or 15 mg.
Wegovy Titration
- Start: 0.25 mg weekly for four weeks.
- Gradual escalation over 16-20 weeks.
- Maintenance: 1.7 mg or 2.4 mg.

Common adverse effects (percent incidence)
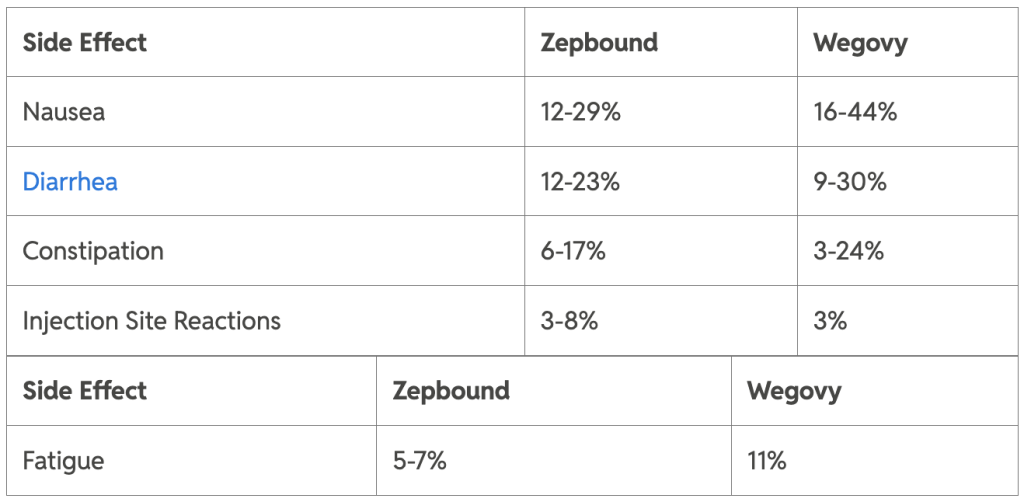
Serious risks include pancreatitis, gallbladder issues, and thyroid C-cell tumors (boxed warning for both). Wegovy shows higher rates of hypoglycemia in diabetes patients.
Patient considerations
Ideal Candidates
- Zepbound: Adults needing >15% weight loss with metabolic comorbidities.
- Wegovy: Patients prioritizing cardiovascular protection or requiring pediatric use.
Contraindications
- Personal/family history of medullary thyroid carcinoma.
- Multiple endocrine neoplasia type 2.
- Severe GI disease.
Novant Health emphasizes comprehensive care. Medications work best with dietary changes and exercise regimens.
Long-Term Use and Discontinuation
- Both medications require ongoing use to maintain weight loss.
- Abrupt cessation may lead to weight regain (70%-80% of lost weight within one year), rebound hunger, and metabolic changes.
- Tapering protocols are recommended under medical supervision.
Lilly's 2025 data shows Zepbound maintains efficacy for two-plus years with continuous use.
This story was produced by SaveHealth and reviewed and distributed by Stacker.



