How spectroscopy is revolutionizing modern research
How spectroscopy is revolutionizing modern research
Virtually every scientific field has made strides by harnessing light to study the properties of substances. This technique, called spectroscopy, has countless research applications, and innovations in spectroscopic technologies are allowing it to make more accurate, efficient and diverse contributions today than ever.
Ocean Optics explores how spectroscopy works, its variations and its applications in the lab and beyond. Discover how spectroscopic innovations will continue to revolutionize modern research.
What Is Spectroscopy?
Spectroscopy is a method for analyzing the interaction between matter and electromagnetic radiation, including light. It helps scientists understand materials by measuring the wavelengths of light that a substance absorbs, emits or scatters. Each material’s molecular structure and composition produce a unique spectral pattern, called a spectrum, when exposed to electromagnetic radiation, so spectroscopy can help identify and quantify materials with precision.
How Does Spectroscopy Work?
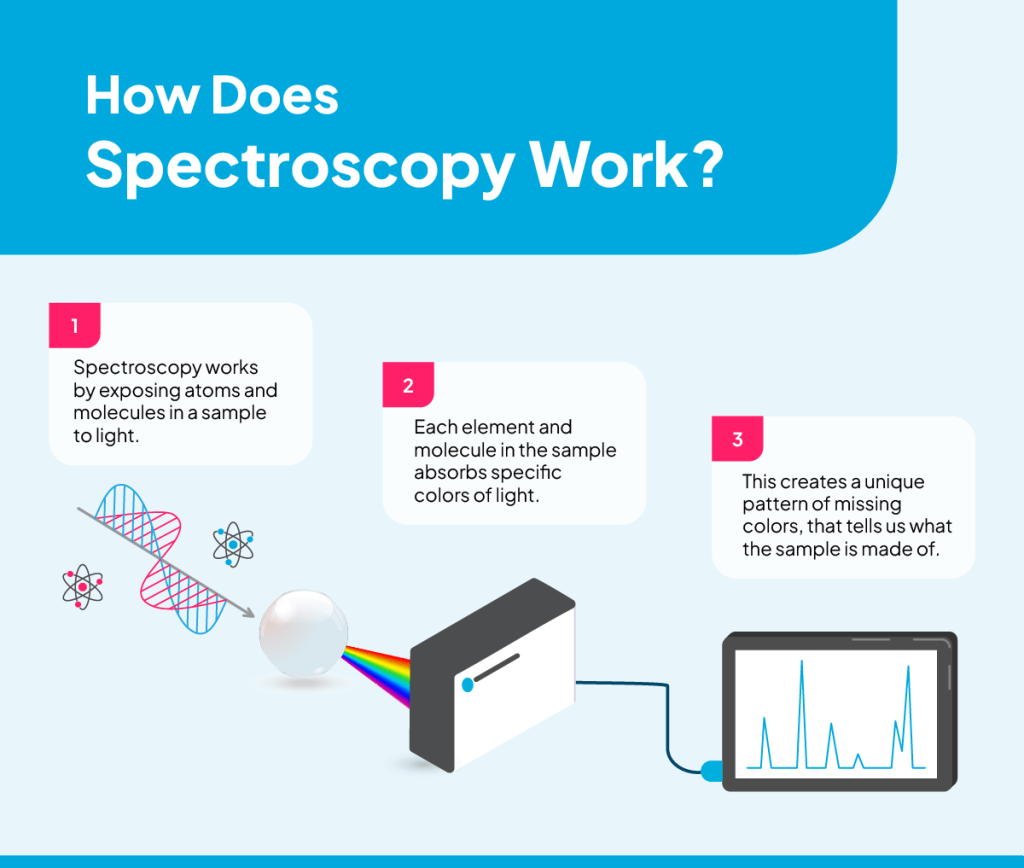
Spectroscopy works by exposing atoms and molecules in a sample to light or other forms of electromagnetic radiation across a range of wavelengths. These wavelengths typically include the ultraviolet (UV), visible and infrared (IR) regions of the electromagnetic spectrum, though gamma rays and radio waves have some applications, too. Each region interacts with matter differently, allowing for targeted testing of specific properties or components within a sample.
The sample’s particles may absorb, emit, transmit, reflect or scatter the radiation energy. Each material interacts with light in a unique pattern of absorbance, emission, transmittance, reflectance and scattering because of its molecular structure, including the arrangement of its electrons and the types of chemical bonds it has. This allows scientists to measure the intensity of light at each wavelength before and after it interacts with the sample.
Detectors are the components within spectroscopy systems that sense and measure the intensity of light after it interacts with the sample and is separated into wavelengths by the spectrometer. The detector also helps convert light signals into electrical signals that scientists can analyze and record. Common types of detectors include photomultiplier tubes (PMTs), charge-coupled devices (CCDs) and photodiodes.
Scientists can visualize the data from their spectroscopic measurements as a spectrum of peaks and valleys, which is like a molecular fingerprint. By studying a sample’s spectrum, we can draw conclusions about its material composition, structure and physical environment.
Types of Spectroscopy
Several types of spectroscopy exist, each with distinct advantages for different applications.
- Absorption spectroscopy: This method measures the light absorbed by a sample at different wavelengths. Scientists use it to identify and quantify substances in a sample based on absorbance and transmittance measurements.
- Emission spectroscopy: This approach observes the light a substance emits when excited by an energy source. It helps in studying atom and molecule energy states.
- Raman spectroscopy: This technique studies how molecules scatter light, providing insights about molecular vibration that inform material characterization.
- Nuclear magnetic resonance (NMR) spectroscopy: This method studies the interactions of atomic nuclei in response to magnetic fields and radiofrequency pulses. It reveals in-depth information about a sample’s molecular structure, dynamics and environment. Organic chemistry, biochemistry and materials science often rely on NMR spectroscopy.
- X-ray spectroscopy: Geologists, materials scientists and environmental analysts use this approach to probe substances’ chemical composition and electronic structure. X-ray fluorescence (XRF) is beneficial for studying elemental composition, while X-ray diffraction (XRD) helps scientists examine crystalline structures.
Though not a form of spectroscopy, mass spectrometry is often combined with spectroscopic methods to achieve more comprehensive and sensitive structural and compositional analyses of complex samples. Where spectroscopy uses light, mass spectrometry uses an electric field, laser or chemical process to ionize molecules, separating them based on mass-to-charge ratio, and detecting them to create a measurable mass spectrum.
A common “hyphenated” technique combining spectrometry with spectroscopy is GC-IR-MS (Gas Chromatography-Infrared Spectroscopy-Mass Spectrometry), which separates compounds in a mixture by gas chromatography, identifies their molecular structures using infrared spectroscopy and determines their masses with mass spectrometry. This technique is used in forensics to identify drugs like barbiturates and cocaine.
What Instruments Are Used in Spectroscopy?
Depending on the application, scientists can choose from a wide array of spectroscopy instruments. There is constant innovation in this area because precise instrumentation is key to obtaining reproducible findings, and efficient instrumentation makes scientific work more productive and affordable. The following are some of the most important spectroscopy instruments.
1. Spectrometers
These instruments measure the intensity of light at different wavelengths, separating incoming light into its component wavelengths and analyzing the resulting spectrum. Types of spectrometers include the following.
- Benchtop spectrometers: These are the traditional, high-precision instruments used in laboratories for detailed analysis and research.
- Handheld spectrometers: Handheld spectrometers are self-contained, battery-powered instruments designed for direct, portable use in the field. They are not typically designed for integration into other systems.
- Miniature spectrometers: These are compact spectrometers, often even smaller than handheld spectrometers, designed for seamless integration into other systems or devices.
- High-resolution spectrometers: These spectrometers distinguish very closely spaced wavelengths. They are ideal for applications requiring intricate spectral observation, like materials science and pharmaceutical analysis.
- Multimodal spectrometers: These devices combine multiple spectroscopic techniques, like Raman and IR spectroscopy, in a single instrument for comprehensive sample analysis.
2. Light Sources
Producing spectra for analysis depends on a source of electromagnetic radiation. The most common are light sources like lamps, lasers and LED lights. Scientists choose a light source based on the stability and wavelength range they need for a given application.
3. Monochromators and Optical Filters
Spectroscopy systems use monochromators and optical filters to isolate wavelengths from the broader spectrum before light interacts with the sample. Monochromators use prisms or diffraction gratings to select wavelengths, while filters block unwanted wavelengths.
4. Fiber Optics
Optical fibers are essential components in modern spectroscopy setups, acting as flexible light guides that connect the light source, sample and spectrometer. They enable remote sampling, modular instrument design and efficient light transmission with minimal loss. Fiber optics make it possible to perform measurements in challenging environments, supporting a wide range of applications from laboratory research to field analysis.
5. Sample Holders
These spectrometer components hold the sample in the light beam’s path during analysis. They are made from materials like glass, quartz or plastic that are transparent to the wavelengths under examination. Sample holders for liquids are called cuvettes. Using a clean sample holder or cuvette in the appropriate material for the wavelengths being studied is essential for accurate measurements of absorbance, emission, transmittance and fluorescence.
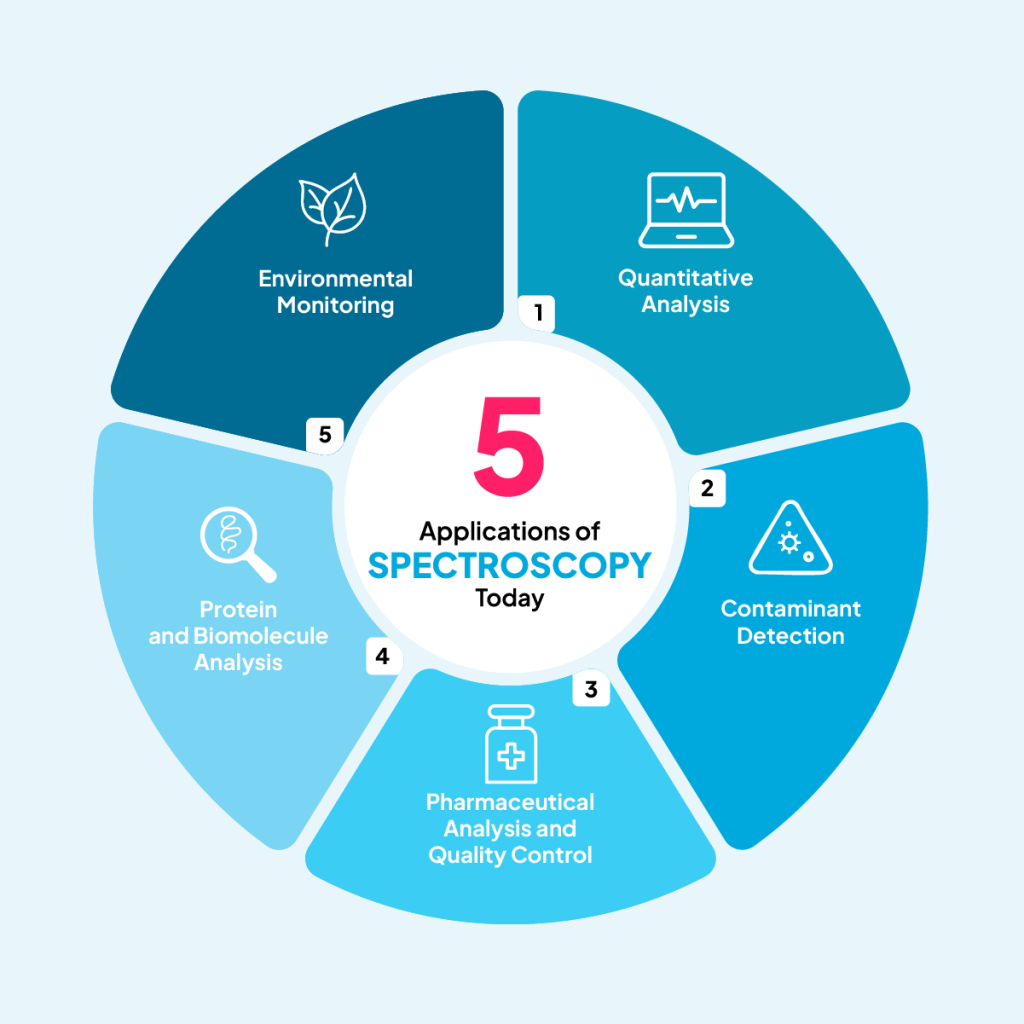
5 Applications of Spectroscopy Today
Spectroscopy’s importance in scientific and industrial research is growing year by year. These are five of the top applications you should know about today.
1. Quantitative Analysis
Spectroscopy has revolutionized quantitative analysis by allowing scientists to measure the concentration of substances in complex mixtures with unprecedented speed and accuracy. By analyzing the intensity of light absorbed or emitted at specific wavelengths, researchers can obtain precise data without destroying the sample. This capability has transformed fields like chemistry, pharmaceuticals and environmental science, making screening, monitoring and compliance more efficient and reliable than ever.
For example, in pharmaceutical labs, spectroscopy allows scientists to measure the exact amount of an active ingredient in a drug batch, ensuring each pill contains the correct dose. In environmental science, it helps researchers track pollutant levels in water samples, supporting safe drinking water and pollution control.
2. Contaminant Detection
Spectroscopy has transformed how we identify harmful substances in our environment and products. Unlike traditional tests that may only measure overall concentration, spectroscopic techniques like IR and Raman spectroscopy can detect specific pollutants or toxins based on their spectra. This means scientists can find dangerous chemicals or impurities, even in complex mixtures like food, soil or industrial materials. Early and accurate identification helps reduce health risks and support regulatory compliance.
3. Pharmaceutical Analysis and Quality Control
Spectroscopy is changing how pharmaceutical companies make and test medicines by enabling real-time, nondestructive checks throughout manufacturing. Techniques like Raman spectroscopy can analyze tablets or powders as they move through production lines, verifying purity without halting production or destroying samples. This approach helps manufacturers catch problems early, reduce waste and protect patients. It also streamlines regulatory approval by providing thorough, automated quality control documentation.
4. Protein and Biomolecule Analysis
Spectroscopy has opened new frontiers in life sciences by enabling detailed, nondestructive analysis of proteins and biomolecules. Absorbance and fluorescence spectroscopy techniques can quantify protein concentration, assess purity and monitor changes in protein structure or interactions.
For example, researchers use absorbance measurements at 280 nm to determine protein concentration, while fluorescence spectroscopy can track conformational changes or binding events by detecting changes in intrinsic or dye-labeled fluorescence. These methods support applications from basic research to drug development.
5. Environmental Monitoring
Modern spectroscopy has transformed environmental monitoring by making it possible to track pollutants, greenhouse gases and other markers on-site. Miniature and handheld spectrometers capture actionable data to reduce pollution and manage ecosystems. This revolution in monitoring empowers scientists and policymakers to make informed decisions, supporting global sustainability efforts.
7 Trends and Innovations in Modern Spectroscopy
Spectroscopy will continue driving advances across multiple scientific disciplines and industries as long as innovation persists in spectroscopy itself. Every year, collaborative research projects between industry and academia accelerate the development of new spectroscopic techniques, expanding the technology’s reach. Here are the top seven innovative trends we expect to shape the future of spectroscopy.
1. Miniaturization
Handheld and miniature spectrometers deliver real-time analysis beyond the lab, enabling rapid testing in forensic, food and environmental sciences. Expect precision in miniature spectrometers to continue improving and support specialized, high-stakes applications like medical diagnostics.
2. AI-Driven Data Analysis
Rapid advances in artificial intelligence and machine learning empower scientists to interpret vast and complex spectral data by automating pattern recognition, accelerating analysis and improving predictions.
3. Hyphenated Methods
Combining spectroscopy with techniques like chromatography or mass spectrometry yields richer data for analyzing complex samples. As integration technology advances, these hybrid systems will become more automated and accessible, making multimodal analyses routine in clinical, pharmaceutical and environmental labs.
4. Ultrafast Laser Techniques
Ultrafast spectroscopy uses femtosecond lasers with pulses lasting just a millionth of a billionth of a second to capture molecular and electronic changes as they happen, providing new, real-time insights into chemical reactions and material properties. By revealing details about processes that were once too fast to observe, ultrafast spectroscopy could lead to breakthroughs in medical therapies, electronics and the efficiency of photovoltaic cells.
5. Hyperspectral Imaging
This technique collects a full spectrum at every pixel, offering detailed spatial and chemical information. Hyperspectral imaging is already revolutionizing agriculture through advanced crop health monitoring and medicine through early disease detection.
6. Surface-Enhanced Raman Spectroscopy
Surface-enhanced Raman spectroscopy (SERS) uses nanoparticles to boost sensitivity, enabling the detection of trace biomolecules, toxins or nanoparticles. SERS supports innovative biomedical diagnostics, food safety testing and nanotechnology research.
7. THz-Raman
Terahertz Raman spectroscopy, also known as low-frequency or THz-Raman, is a form of Raman spectroscopy that measures vibrational modes in the terahertz (THz) frequency range. This technique provides insights into lattice vibrations, crystal structure and polymorphism in materials.
THz-Raman is valuable in pharmaceutical research for distinguishing between different solid forms of a drug, and in materials science for characterizing polymers, minerals and nanomaterials. Because it is nondestructive and requires minimal sample preparation, THz-Raman is popular for quality control, counterfeit detection and advanced research.
Light Matters With Spectroscopy
By illuminating the interactions between light and matter, spectroscopy has become an essential tool for modern research. Its ability to deliver rapid, accurate and detailed analyses is revolutionizing laboratory research and real-world industry applications. As advances in instrumentation and data analytics continue, the latest developments in spectroscopy will unlock even more discoveries, helping scientists solve complex problems and improve lives worldwide.
This story was produced by Ocean Optics and reviewed and distributed by Stacker.